I Overview
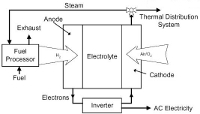
Fuel cells use electrochemical processes to generate power. These processes are very similar to those that allow dry cell batteries to function.
Inside a fuel cell are two electrodes, the cathode and anode, separated by an electrolyte. Hydrogen enters at the anode, and oxygen enters at the cathode. A catalyst helps split the hydrogen into protons and electrons. The protons and electrons both move to the cathode, taking separate paths. The protons pass through the electrolyte, and the electrons travel through an external circuit connected as a load. When the electrons arrive at the cathode, they combine with the protons and oxygen, producing only water and heat as byproducts.
The hydrogen fuel source can be produced by a number of schemes, including natural gas reformation. Some fuel cell packages provide the natural gas reforming system, allowing long-term unattended operation on natural gas and ambient air.
II Applications
Potential distributed generation applications for fuel cell systems include cogeneration, premium power, remote power, specialty applications, grid support, peaking power and microgrid applications.
III Equipment Options
Fuel cells are generally categorized by the type of electrolyte they use. The five main types are proton exchange membrane (PEMFC), alkaline (AFC), phosphoric acid (PAFC), molten carbonate (MCFC), and solid oxide (SOFC).
IV Resources
1. Equipment Manufacturer Database
2. Distributed Generation Consortium
V Photo